Uncomplicate Compliance: Modern Requirements’ Solutions For Medical Device Makers
More than ever before, medical device manufacturers are hard-pressed to deliver products that enable faster, cost-effective and more efficient patient care. To remain competitive, companies must successfully locate their sweet spot that brings down overhead and boosts patients’ safety and survival rates.

Requirements, Compliance, and Medical Devices
In a heavily regulated industry such as health care, bundles of red tape that come with compliance can seriously weaken the manufacturers’ competitive edge. In other words, The Food and Drug Administration’s byzantine approval process for new devices can hamper innovation and impede access to improved health care.
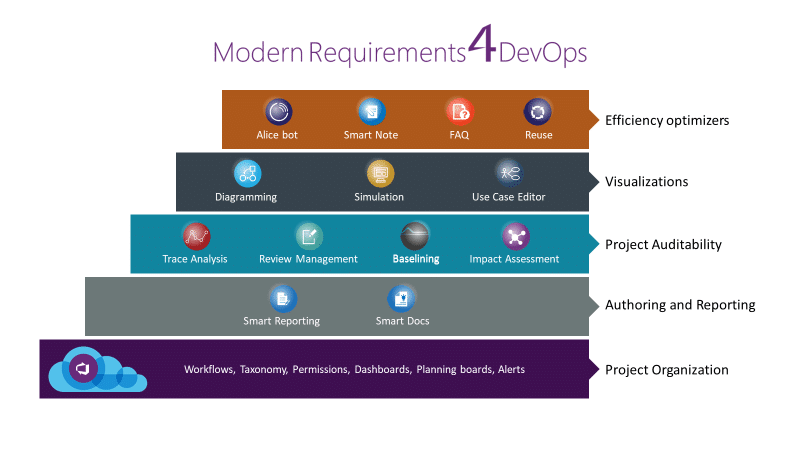
We understand the unique – and often burdensome – complexities that come with industry regulation. And the way they impact your organization. Rapid technological changes offer unprecedented advantages but can also trigger a few irksome challenges. Slow compliance, long development cycle times and drawn-out value delivery are just a few of such challenges. However, many firms who use Modern Requirements4DevOps (aka MR4TFS), as an embedded extension of their MicrosoftAzure DevOps Server, implementation have seen these problems greatly reduced.
The following list summarizes only several of the benefits that Modern Requirements’ clients (Siemens, Becton Dickinson and Varian Medical Systems, among others) have experienced after implementing Modern Requirements4DevOps (aka MR4TFS). These benefits are crucial in helping you ensure that all of the regulatory requirements within your processes are met, resulting in quicker complete compliance, slashed product development cycle times and faster value delivery.
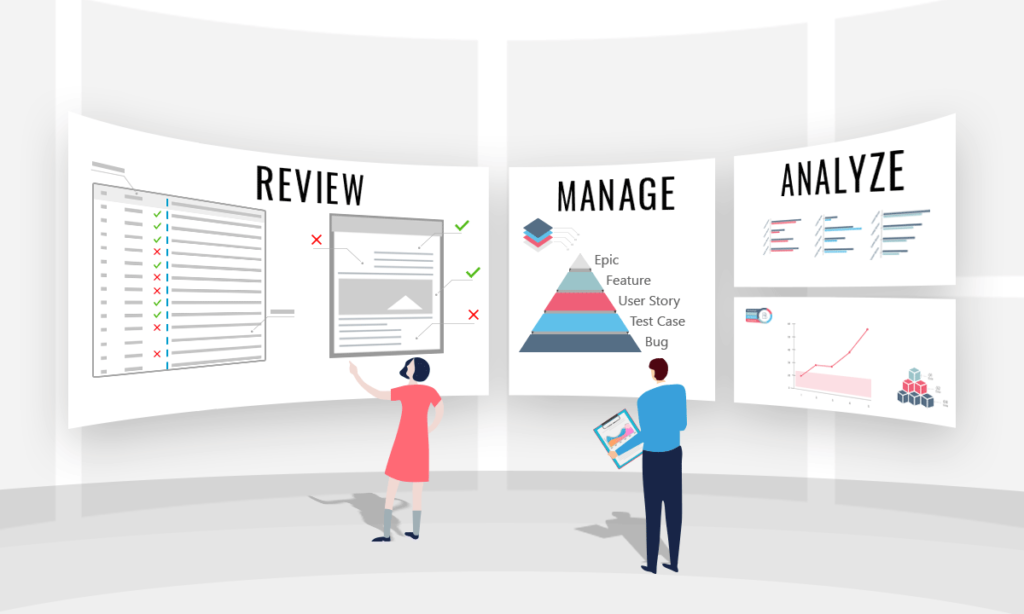
The Benefits of MR4DevOps for Medical Device Companies
Collaborate To Obtain Approvals
Schedule peer or formal reviews and boost participant input and integrate reviewers’ and approvers’ comments on a continued basis during your project. Modern Requirements4DevOps (aka MR4TFS) lets you connect e-signatures to selected reviews and achieve full 21 CFR Part 11 compliance.
Auto-Generate Reports
Report generation and spreadsheet use are keystones of your daily project activities. They facilitate communication and collaboration during and after product development. With Modern Requirements4DevOps (aka MR4TFS), customized reports are available on-demand, wiping out repetitive tasks like copy & paste and file transfers.
Baseline Requirements
Capturing the state of a product’s requirements at a specific point in time is essential to fulfilling your regulatory reporting. Plus, being able to compare various baselines to see what has changed helps you manage your product development more efficiently. If a particular product version fails, you need to rollback requirements to a previously working state. Modern Requirements4DevOps (aka MR4TFS) enables you to manage versions, branch and merge and comply with reporting demands.
Reuse Requirements
Duplicate work item information as you move from one project to another. This way you build an enterprise library of reusable specifications for application in various projects. Reusing requirements is made uncommonly simple with Modern Requirements4DevOps (aka MR4TFS): we provide you with a choice of 19 categories of non-functional requirements, such as safety and security, complete with a detailed list of questions to help you define these exacting types of requirements.
Identification of Gaps
Connect and granulize sophisticated requirements down to the finest detail and ensure that adequate coverage exists for all key requirements and tests. Traceability is so much more than a fixed picture of your requirements – it’s a dynamic tool that warns you about any gaps that pop up in the system and it helps you make sure all data elements are linked accurately.
Trace Reporting
Create sub-system requirements, incorporate relevant risks, hazards and tests, and complete appropriate validation and verification in time for your product release. Follow our automated reporting approach, leveraging horizontal or intersection trace matrices – and forget about the mind-numbing manual procedures that are often followed by default.
Managing Change
Use unalterable audit trails to track all data changes being made during the project – and in real time. Let the system auto-identify potentially impacted or “dirty” requirements that result from a proposed change. Export changes such as timestamps and associated users from Modern Requirements4DevOps (aka MR4TFS) quickly and seamlessly. And, select impact assessment to give you vital intelligence on the repercussions of anticipated changes visually, in a mind map. Then create estimates and tasks to manage the change.
Hazardous Risk Reporting
Generate a hazard risk report to identify factors that pose a threat to your project. This feature enables you to analyze and evaluate risks of a specific hazard and establish suitable ways to mitigate it. Or implement risk-control measures if the hazard cannot be removed.
Ease of Authoring Requirements
Bridge the gap between document and information management by authoring requirements in a document view online. Add detailed tables and leverage rich text and images to provide rigor to your product’s requirements, while simultaneously creating work items in the database. Create authoring templates per product, save your work in folders and reuse the templates as needed on new projects.
Wrapping Up
Meeting the regulatory requirements is often a gnarly, cumbersome process. If not managed properly, compliance can weigh down your company’s resources and pose an unnecessary corporate enterprise risk. But it doesn’t have to be this way. By implementing Modern Requirements4DevOps (aka MR4TFS), you can simplify compliance by automating tasks across the product development lifecycle. Once you put Modern Requirements4DevOps (aka MR4TFS) into action, you’ll soon wonder how you managed without it.
Give it a try in the cloud today.
Author: Bob Savelson
Time to Read: 8 minutes
Related Articles
Request a Demo!
- Schedule a demo with one of our trained product experts.
- Receive a personalized demo that mimics your team's process
- Engage our experts on topics such as workflow or best practices.

Reduce UAT Efforts
50% Reduction in UAT efforts

Proven Time Saving
80% time saving on creating Trace Analysis

Streamline Approvals
Significant reduction in approval delays

Increase Performance
50% requirements productivity improvement

Reduce Rework
10-fold reduction in development rework
Simplify Compliance
40% reduction in compliance reporting efforts


