Mastering Medical Device Software Development with Requirements Management
- Arunabh Satpathy
- December 12, 2023
- 9 minutes
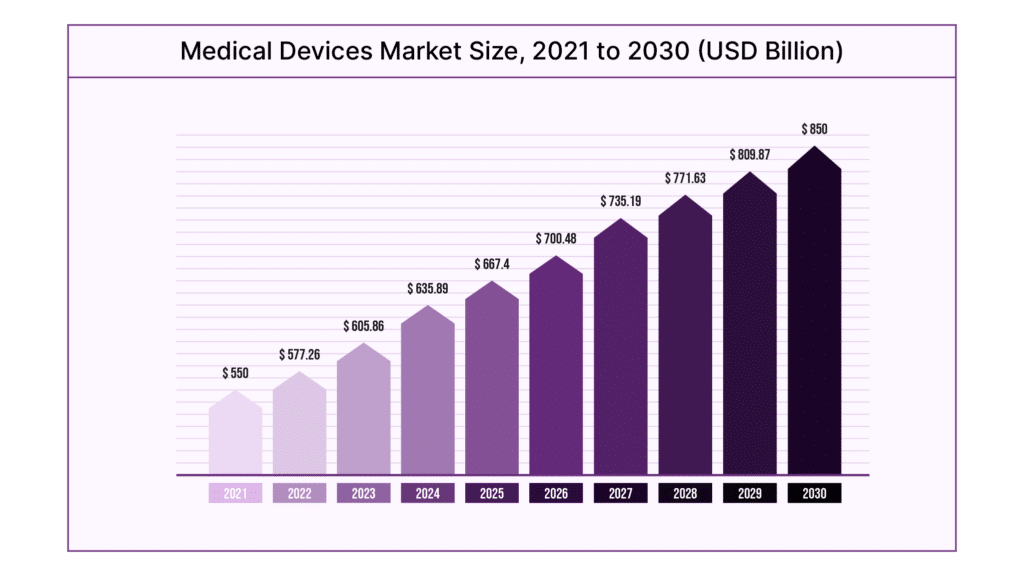
Medical Software Development is the process through which companies develop apps for both patients and medical professionals. A critical part of success in medical device software development is robust requirements management. These tools help teams balance user needs, business needs, and strict regulations. As the industry becomes more competitive, balancing these needs is getting harder. According to Statista, the medical devices market is projected to grow to $609 billion in revenue by 2028, up from $471 billion today.
As innovation drives the industry forward, the need for precision, compliance, and efficiency is increasing. This article covers common challenges in the medical software industry and how requirements management solutions can help you.
1. Challenges in Medical Device Software Development
Medical device companies face different and greater challenges than non-medical device companies. Regulatory hurdles, the stakes for end-users, and the complexities of project management pose significant roadblocks. Any software that helps you navigate these challenges also becomes a healthcare risk management software. Here are some common challenges companies face in medical device software development:
- Product complexity: Because stakeholders need high-grade hardware combined with sophisticated software, medical device development has become increasingly complex. This means making medical devices is increasingly fraught with risks and difficulties.
- Regulations: Because the field of medicine aims to save lives, regulators are constantly increasing compliance standards, including for medical device software. This is a daily challenge for companies designing medical devices. For instance, the FDA has recently been reviewing and authorizing AI/ML enabled devices, which is an entirely new category of devices.
- Patient Data: Related to regulation is the central role that patient data plays in healthcare software. A person’s medical records and metadata are highly sensitive pieces of information. Companies that design medical device software have to deal with massive amounts of patient data, while maintaining data quality, scalability, and access.
- UI/UX: A software-enabled medical device may be used by many stakeholders or only a few. The common thread between them all is the need for genuinely friendly and easy-to-use software. While many medical devices serve a crucial need for patients, they fail to provide a good user interface to users, which can have catastrophic consequences.
2. The Need for Requirements Management Tools
Medical device software is a critical component in modern healthcare solutions. Its development demands a meticulous approach to ensure functionality, safety, and regulatory compliance. Requirements Management tools serve as the bedrock for success, providing a structured framework to capture, analyze, and trace every aspect of software requirements.
The traditional method of requirements management in healthcare development is unsuited for medical device software development. With this method, hardware and software teams would meet these needs separately after agreeing on stakeholder needs. But they used different tools and methodologies, leading to traceability issues and expensive rework.
This leads to a long process of finding and fixing problems between the two teams and can take up a lot of time that could have been used to make the device better.
To manage product complexity, user interfaces, regulations, and patient data, requirements management tools are necessary. They streamline development and deployment of software and mitigate risks during the development of medical devices and their software. The best requirements management solutions leverage widely used existing platforms like Azure DevOps and turn it into a single source of truth. They also manage healthcare risk management by extension.
3. Aligning with Regulatory Standards
In healthcare, meeting regulatory needs is key to medical device success. It is estimated that the global market has two million medical devices in total, divided into approximately 7000 device groups. These devices must meet global, local, and category specific regulatory standards.
Regulatory bodies like the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe set these standards. In the US alone, the FDA requires medical devices to satisfy at least seven basic regulatory requirements. These standards cover a wide range of medical device aspects, from design to post-launch surveillance. An ordinary blood glucose monitor may have to adhere with the following (non-exhaustive) list of standards:
| Function | Regulation (if relevant) | Compliance Standard | Level |
| Infection Control | CDC guidelines on infection control during blood glucose monitoring | CLIA Certificate of Compliance (CoC) or Certificate of Accreditation (CoA) | National (US) |
| Device Design and Use | FDA guidelines for self-monitoring blood glucose test systems | ISO 15197 | National (US) |
| E-Signatures | Title 21 of the Code of Federal Regulations, established by the FDA. | CFR Part 11 | National (US) |
| Risk Management | N/A | ISO 149715 | International |
| Software Lifecycle Processes | N/A | IEC 623046 | International |
| Health Software Safety | N/A | IEC 82304-17 | International |
| Medical Electrical Equipment Safety | N/A | IEC 60601-18 | International |
Requirements management tools play a crucial role in aligning medical products with standards. A team must document, analyze, trace, and prioritize requirements they must satisfy. By effectively managing requirements, medical device companies can ensure that their products not only meet regulatory standards, but also deliver safe and effective solutions to healthcare providers and patients.
4. Medical Device Software Development with Requirements Management
Precision is non-negotiable in the medical field, where the stakes are high, and accuracy is paramount. Requirements Management tools enable development teams to articulate and document software requirements with meticulous detail.
When creating medical device software, teams may use multiple features that requirements management tools provide, like:
- Requirements Elicitation/Gathering: This involves collecting requirements through discovery, analysis, FAQs, elicitation techniques, and now AI.
A practical example of a requirements elicitation technique is interviewing stakeholders. The most important factor in elicitation through interviews are the questions you ask. Elicitation is best done early in the prototype design. A pre-existing and editable batch of FAQs can help you gather the right questions for eliciting. An added AI tool can also generate project-specific questions by ingesting raw requirements data.
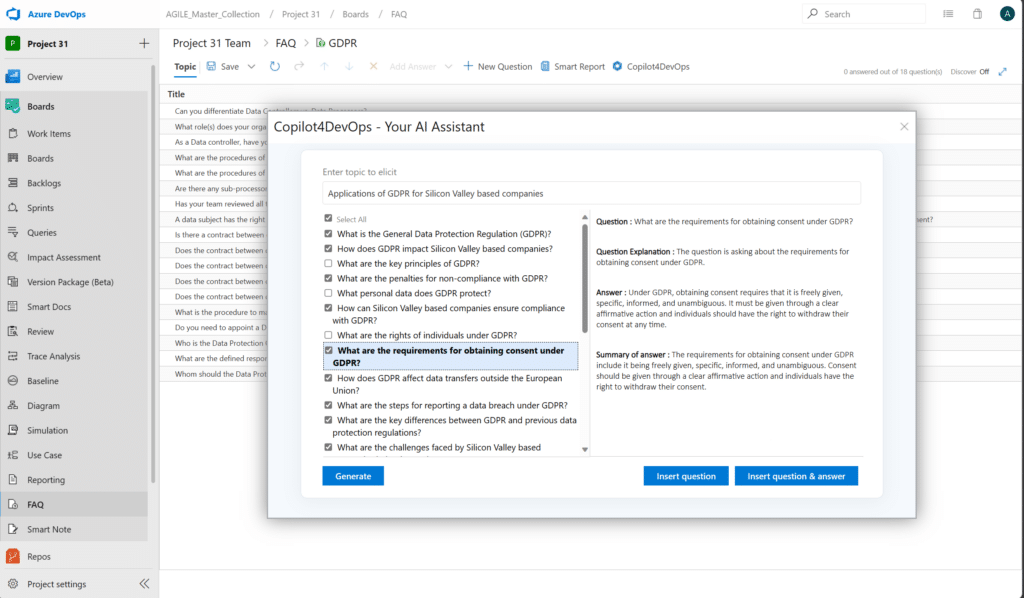
- Requirements Documentation: This includes documenting the planning, development, verification, validation, and changes to your requirements. For medical devices (including their software development), include information for every possible element. Some of this documentation is scrutinized by regulatory bodies, so comprehensive testing data and evidence is necessary.
Many errors in documentation may creep in between tight deadlines and high-stakes competition. More than a third of software engineers consider poor documentation one of their biggest challenges. This is more critical for medical device software development.
Easily creating structured Word-like requirements documents within an ALM like Azure DevOps can help reduce these errors.
- Traceability: Tracking the relationships between work items throughout development is also important in medical device software development. In medical device development, there are you must account for:
- Traceability between product requirements and tests
- Traceability between test measurement results and national standards
- Traceability between devices and its components from manufacturers to the end-customers
The complexity of harmonizing hardware and software development can create errors. It’s easy to lose track of how tasks are related. Creating traceability matrices to trace requirements from end-to-end can help reduce these errors. It also helps project efficiency, component testing, impact analysis, and team communication.
- Report: Creating standard requirement reports, dashboard views, and device specifications to track project progress helps keep your project on track. Reporting and accountability are essential to the regulation-heavy healthcare technology space. Agencies and teams often need to know how a piece of the project is going. Medical device companies must report on establishment registration, device listing, premarket approval, clinical study exemptions, quality system regulations, labeling requirements, and adverse event reporting.
In such cases, it is helpful to output reports of anything and everything from your project in one click.
It’s even better if you can output documents as PDF, Word, or HTML.
Modern Requirements4DevOps is a solution designed to streamline the requirements management process in medical device software development. Its key features include traceability, collaboration, and change management, which enhance project efficiency and ensure alignment with regulatory standards. It also has a groundbreaking AI tool called Copilot4DevOps, which increases requirements quality through elicitation, analysis, and a lot more.
By leveraging Modern Requirements4DevOps, healthcare organizations like Bayer and Red Cross are ensuring better regulatory compliance, project efficiency, and reduced development costs. Here’s how you can use it too.
5. Mastering Requirements Management
Effective requirements management is crucial in medical device software development. Though requirements management tools are the best way to level-up your project, there are also some best practices that should apply regardless of the tools you use:
- Define Clear Requirements: Requirements should be clearly and concisely written. For additional help, you can use AI quality analysis to rank your requirements on the 6Cs of writing.
- Maintain Traceability: Use traceability matrices to track the origins of requirements and changes to requirements over time. It’s essential to demonstrate compliance with local and international standards.
- Collaborate and Communicate: Requirements management is a collaborative process involving many stakeholders, from end users to licensees. Communicate regularly with all stakeholders to ensure the product is aligned with expectations.
- Manage Changes Effectively: Changes in requirements are inevitable. A good system will help you track all changes across medical device software and hardware.
- Focus on Healthcare Risk Management: Identify potential risks associated with the medical device and implement measures to mitigate these risks. includes using requirements management tools. But it also means taking extra care to ensure compliance with standards like ISO 14971 in a high-stakes world like healthcare. People’s lives are at risk.
- Invest in Training: AI tools are easy-to-use, but training is still essential to extract the maximum from them. Companies would be wise to invest in employee training for AI. The Workmonitor Pulse research by Randstad discovered that only 13% of employees have received any training in artificial intelligence (AI) in the past year.
6. More Than Just Medical Device Software Development
As the medical device market grows, the difficulty of balancing hardware, software, and regulation will increase. A robust requirements management tool is your ally when managing these complexities.
Newly by artificial intelligence, these tools streamline development, ensure regulatory alignment, and reduce risks. They can do this for both medical device software and hardware. As the sector evolves, these tools will be pivotal for success, driving innovation, and leading to better patient outcomes.


