Facilitate the Medical Device Design Controls with Modern Requirements

Constant development and innovation in medical devices is critical to ensure personal health and safety. Due to the nature of medical devices and the strict regulatory compliance needs, requirements management is deeply related to the success of any type of medical device.
In this article, we will explore how to manage the medical device design controls more efficiently by using Modern Requirements.
Understanding Medical Device Design Controls
Design Controls represent a mandated approach to show many layers of documentation, the purpose of which is to ensure that medical devices meet user needs, intended uses, and specified safety requirements.
The range of medical devices regulated is huge, including pacemakers, cardiovascular stents, respiratory ventilators, and relatively simple devices such as patient scales and elastic bandages. In the US, Design Controls are required for all Class II and III medical devices, which normally indicate a medium and high level of risks. Likewise, ISO 13485 mandates design controls.
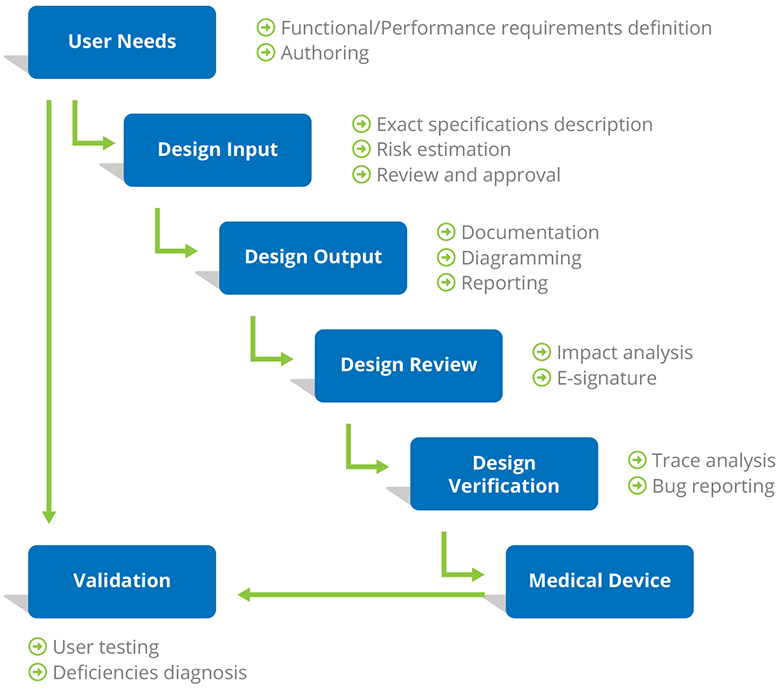
“No sufficient design controls” is proved as one of the major causes of device recalls, and therefore you will need a professional requirements management tool for authoring and documenting requirements during the entire development lifecycle.
How could Modern Requirements help?
Modern Requirements is a world-class requirements management tool and has been a market leader for over 10 years. With clients such as Philips Healthcare, Siemens Healthineers, Becton Dickinson, and Varian Medical Systems, we keep absorbing suggestions and feedback and improving our capabilities of assisting our clients in medical devices regulatory compliance in each possible way.
As the only fully embedded solution for Azure DevOps Server (TFS) and cloud-based Azure DevOps Services (VSTS), Modern Requirements extends Microsoft’s leading ALM tools to create a one-stop shop solution for your requirements management needs.
The system capabilities described below are crucial in helping to ensure that all of the regulatory requirements within your design controls processes are met, resulting in quicker complete compliance, slashed product development cycle times, and faster value delivery.
Requirements authoring
Over the past decade, we have consistanly heard from our clients that using Modern Requirements for requirement authoring is an easy and pleasant process.
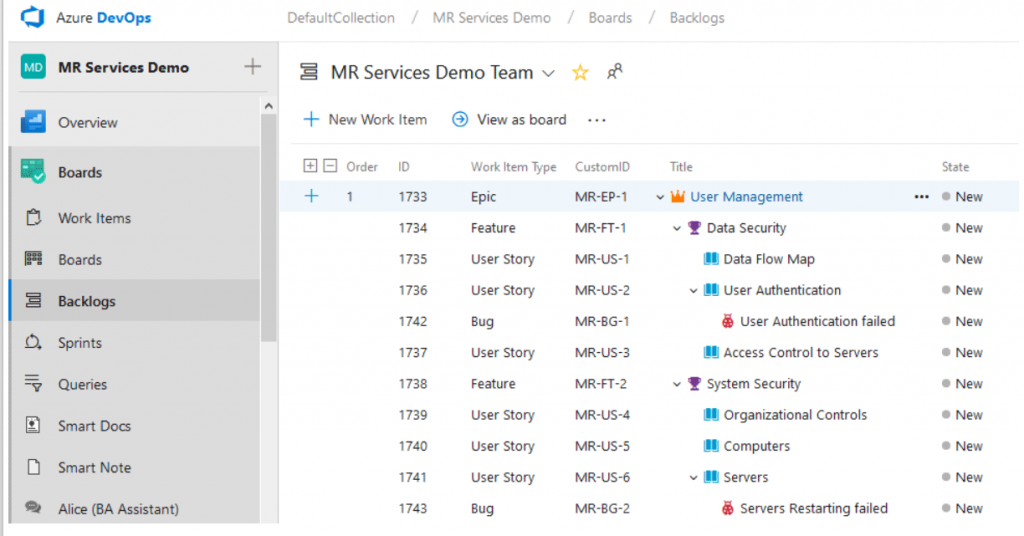
Every project is unique. There is no universal process template for all projects. With ModernRequirements4DevOps, you decide the requirement Work Item types, their hierarchical relationships, and what fields to include in each Work Item type that best fit your project and team; no matter whther you subscribe to Agile, CMMI, Scrum, or a custom hybrid type of methodology.
Normally we see over thousands of requirement Work Items in a single medical device design project. You can use the CustomID feature to automatically categorize your requirements and help your team identify and use them more efficiently.
Physicians, surgeons, technicians, and nurses, whoever you invite to participate in the design process of your new device, now can join the project discussion or even create requirements directly through email without a Modern Requirements license! Review our Email Monitor feature to know more about how to join requirements authoring for external stakeholders.
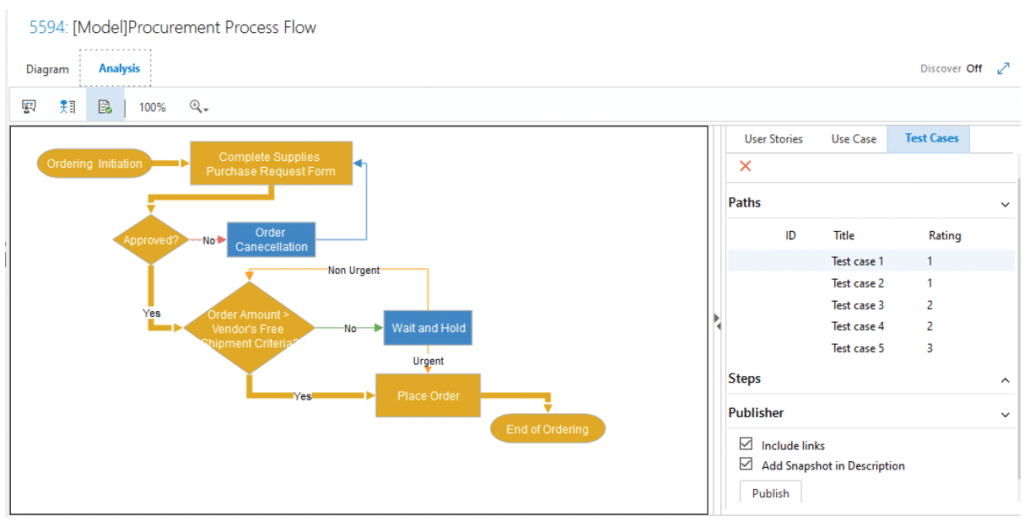
How about diagramming? Diagramming is another way to create requirements in your project. You may need a diagram to describe the relationship between Design Controls elements or another diagram to visualize the data flow. What’s different in Modern Requirements is that we have fully integrated the diagramming solution with your Requirements Management process! With just one click, the system could automatically generate User Stories, Use Cases, and Test Cases simply by extracting information from the diagram you created within our module. With provided stencils and an intuitive positioning guide, let us help you integrate Diagrams fully into your requirements authoring process.
The system could detect all possible routes in your diagram and generate requirements accordingly. All you need to do is to make a decision to publish them or not.
Hazardous risk scoring
Still using tools outside your project environment to calculate hazard or risk scores? Now use the MatCal functionality for automatic Risk Scoring in the same project environment within ModernRequirement4DevOps.
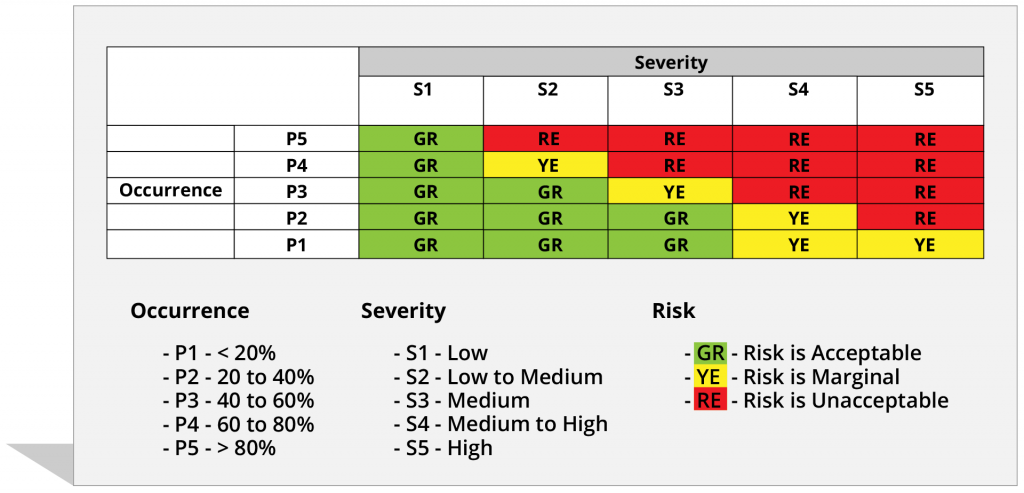
Risk scoring is a process of calculating a score which could indicate how severe a risk is, based on associated factors. It is one of the most critical parts of risk and hazard management in medical device compliance. With the help of MatCal and our reporting tools, you can generate a hazard risk report to identify factors that pose a threat to your project. This feature enables you to analyze and evaluate risks of a specific hazard and establish suitable ways to mitigate it. Or implement risk-control measures if the hazard cannot be removed.
Risk rating is automatically assigned according to Severity score and Occurrence score
Documenting and Reporting
Compiling for the Design History File (DHF)? Documenting all user needs, design input, design planning, design output can be overwhelming. Smart Docs, one of MR4Devops client’s favorite features, allows you to display your product’s requirements in a document view. You can add detailed tables or built-in diagrams and leverage rich text and images to provide rigor to your product’s requirements, while simultaneously creating Work Items in the database. Or simply drag and drop some of your existing requirements to any individual document where you need them.
Modern Requirements4DevOps wipes out the “version” problems you might have experienced before. Changes you make to a Work Item are synchronized everywhere it’s used immediately, no repetitive tasks like copy & paste and file transfers are required anymore. This feature allows you to access your Work Items in multiple ways, either editing your requirements in the Backlog or Work Items view where you could see all your requirements of the current project, or just focusing on a subset you are working on in a Smart Document view.
A sample Smart Document with a few Work Items
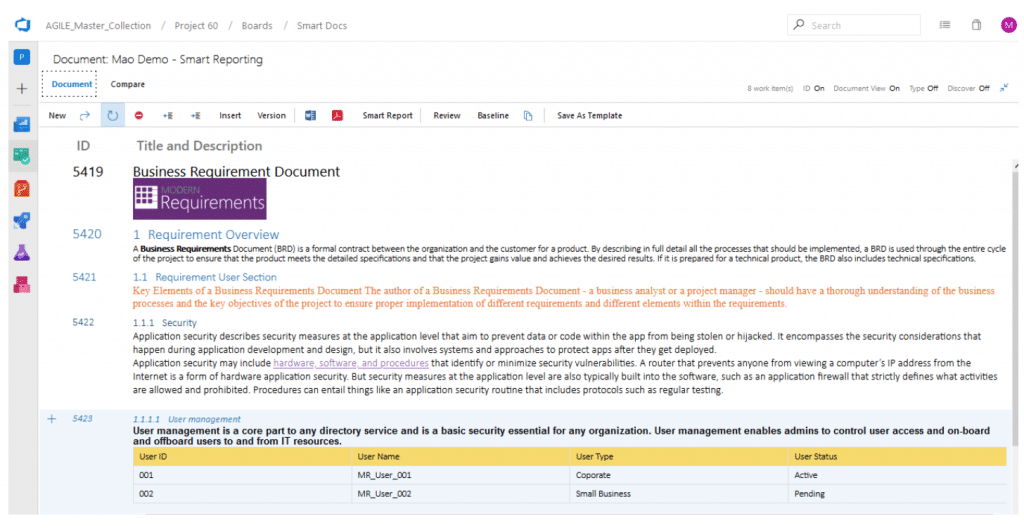
Finally, you can export your documents in Word, PDF or HTML format. Our Smart Report functionality offers more than just a plain export activity – customized reports are available on-demand. In addition to customizing the display format, you can add static content and your company logo or watermark to a built-in report template and reuse it anytime you want.
Change Management
Design control is not a “once and done” process – it applies to modifications or improvements to every stage of the design processes. In Modern Requirements, every change you make to an individual Work Item is captured as a revision of it.
Compare historical versions of a Work Item to track what has changed
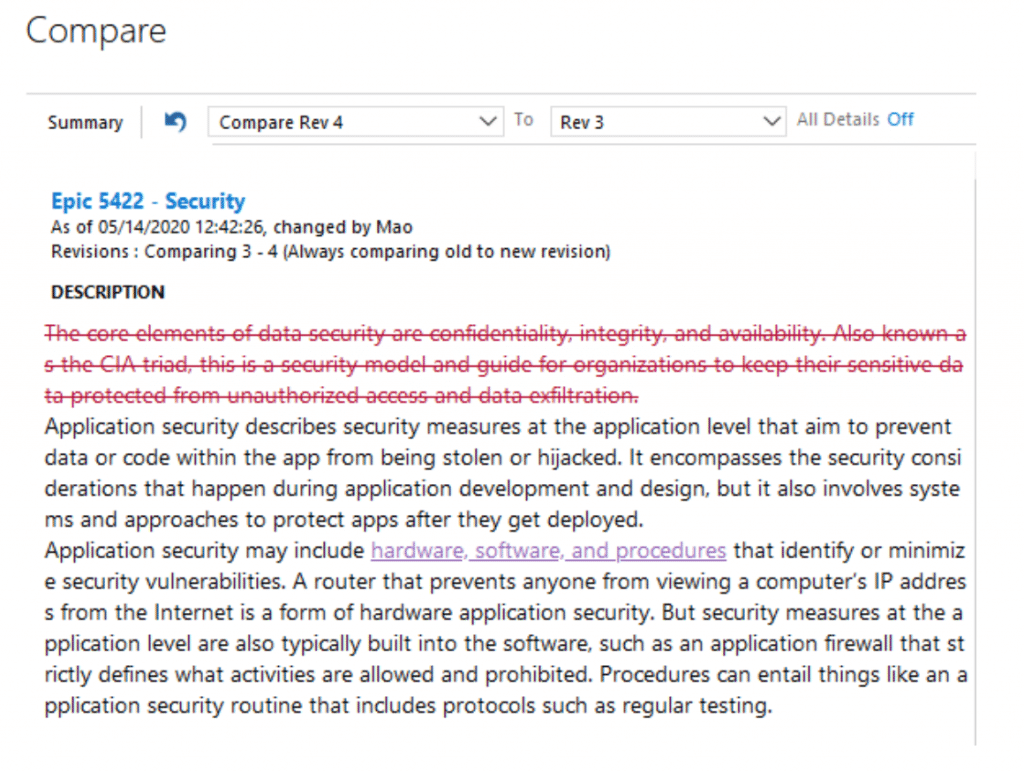
Plus, we have a variety of functionalities to keep track of every major and minor change along the road.
Baseline
Capturing the state of a product’s requirements at a specific point in time is essential to fulfilling your regulatory reporting. Plus, being able to compare various baselines to see what has changed helps you manage your product development more efficiently. If a particular product version fails, you need to rollback requirements to a previously working state. Modern Requirements4DevOps enables you to manage versions, branch and merge and comply with reporting demands in our Baseline module.
Baselining different versions of your design allows you to show the evolution of the design, which can be useful for testing failure investigation. Such records can also prevent repeat errors for the design of future similar products.
The overview of comparing two baselines of the same set of Work Items
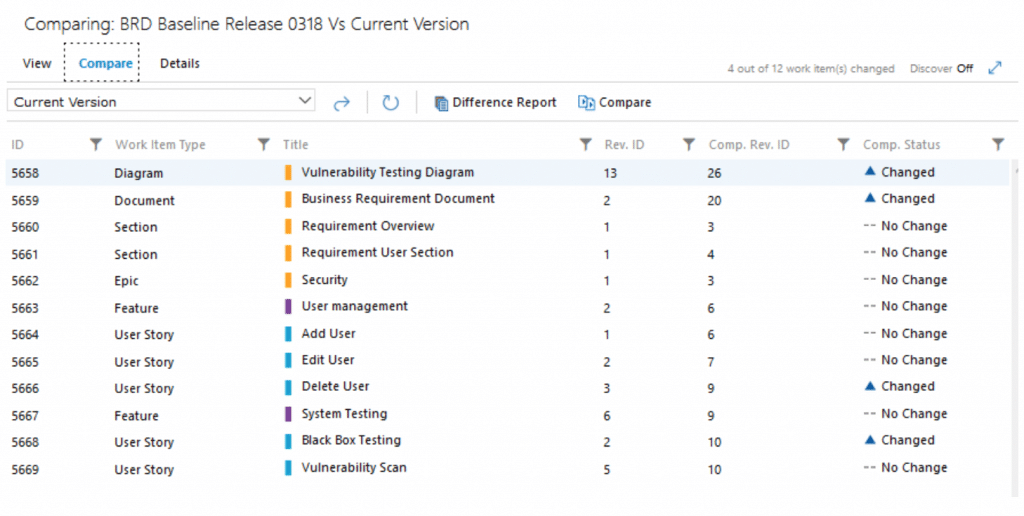
Impact Analysis and Suspect Link
Let’s assume a scenario here. As a pacemaker manufacturer, you are working on finalizing the design requirements for a new pacemaker model. Then your team decides to update the user needs based on suggestions from the nation’s Cardiovascular Health Association. Imagine how many downstream Work Items needed to be reviewed, modified, and documented?
First, the Impact Analysis feature allows you to manage the impact of change by viewing all suspect requirements linked to the changing requirement. Doing so enables you to estimate the impact of each change, and create tasks to complete the work – vs. having to examine each Work Item individually at present.
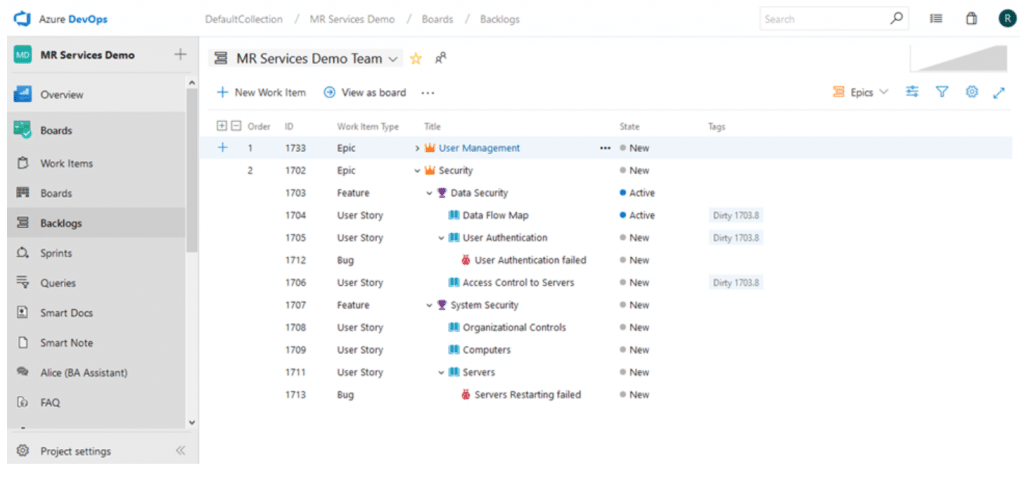
Besides proactively reviewing the impact of related Work Items, the Suspect Link (formerly called Dirty Flag) functionality will mark linked Work Items as “dirty”. This allows your team to easily identify if a requirement that matches a certain set of criteria (which you specify) changes and reminds you to examine associated requirements once before proceeding to the next step.
Work Items were marked as “dirty” since a change condition you have previously defined has been met
Review and E-signature
Review and approval are important parts of the compliance. Both 21 CFR part 11 FDA and ISO 13485:2016 require formal reviews on a regular basis during the development process and all reviews must be documented. The documented results become a part of the DHF. FDA also requires you to add an independent reviewer without direct responsibility for the design stage being assessed.
The Review module of Modern Requirements4DevOps provides access for even external reviewers and approvers to review, comment, and approve your design requirements. In our platform, you can connect e-signatures to selected reviews to achieve full 21 CFR Part 11 compliance.
With just a one-click effort, all historical review results could be generated in an Audit report in PDF or Word format.
Trace Analysis
How to keep track of user needs, hundreds of specifications, and their associated verification and validation status? This is the most asked questions we received from our clients producing Medical Devices.
We offer two types of requirement Traceability Matrices.
- Intersecting Matrix. A 2X2 cross- reference chart between any two types of Work Items. It helps confirm 100% Test coverage, which is a measure of the proportion of the project exercised during testing. Test coverage is basically a quality check on test cases, and we can increase our test coverage by creating additional useful test cases and omiting unnecessary test cases.
An Intersection Traceability Matrix between User Stories (design input) and Test Cases (design output).
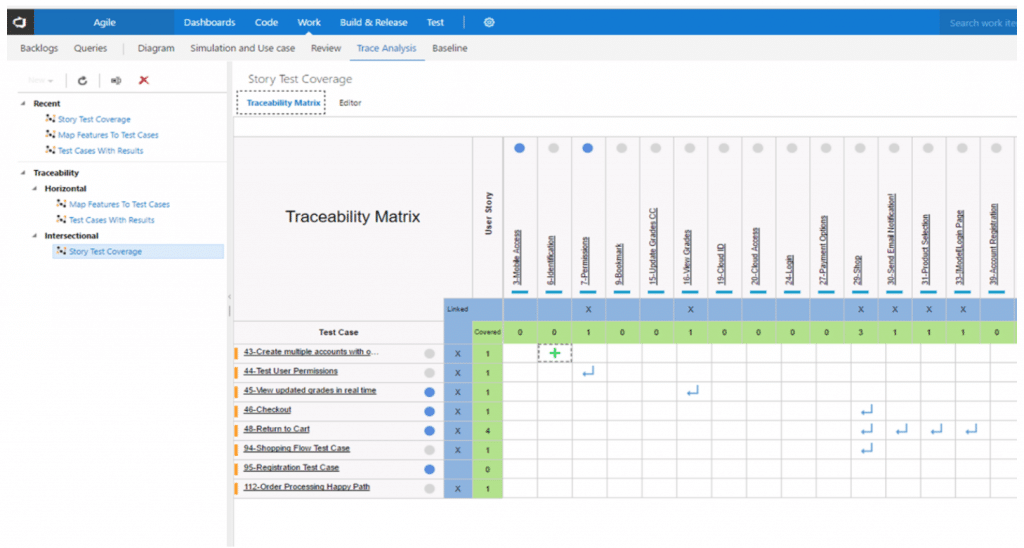
- Horizontal matrix. This matrix maps Product and Technical Requirements with Test Cases and Defects in a linear format and allows for easy identification of linked requirements by finding the specific Test Case. It also helps determine number of tests required, types of tests required, and whether these tests can be automated, done manually, or re-used. Once we figure these factors out, we can yield the best possible test case and help provide the overall defect status through logs of defects.
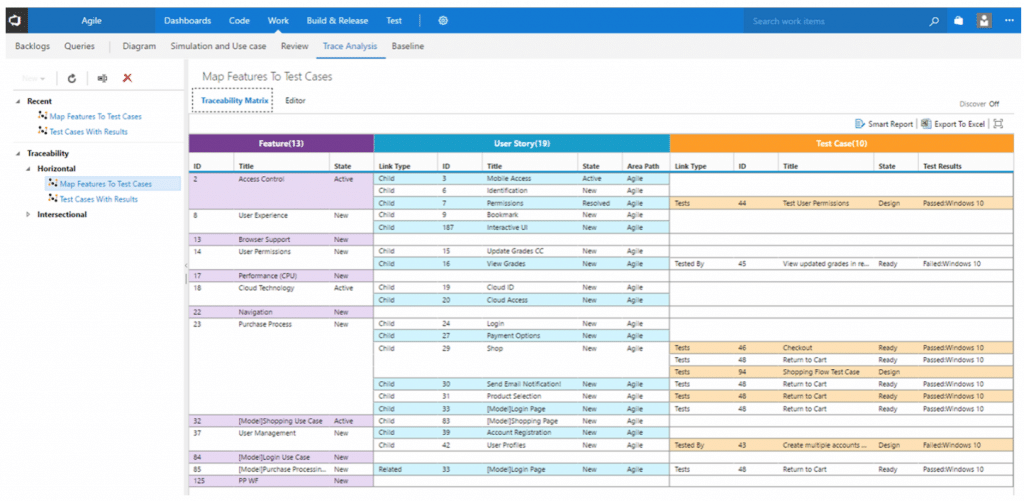
A Horizontal Traceability Matrix is also helpful in providing visual progression to ensure that no functions/requirements are missed when testing. Lastly, a Horizontal Traceability Matrix can help estimate the impact of reworking test cases, conducted by a QA team.
With Modern Requirements, you can save 80% + of your time in creating Trace Analysis. Your Traceability Matrix is always fully configurable and up-to-date.
Final Thoughts
Meeting regulatory requirements is often a gnarly and cumbersome process. Regulators set the expectations but don’t provide the answers. If managed properly, your medical device design control process can be efficient, accurate, and pleasant.
By implementing Modern Requirements4DevOps, you can simplify compliance by gathering all key pieces of information into a single location and leveraging the wisdom we have been accumulating for years to help you take control of your device development processes.

